Here at Blueprint Medicines, we know that advancing patient outcomes relies on more than clinical data alone. We embody this belief every day, including supporting healthcare professionals as they make important treatment decisions for their patients.
Our approach is simple and integrates patient experiences from the start. The outcomes of this work are setting a new standard for clinical success and can have significant impacts for those faced with diseases such as systemic mastocytosis (SM).
The power of real-world insights
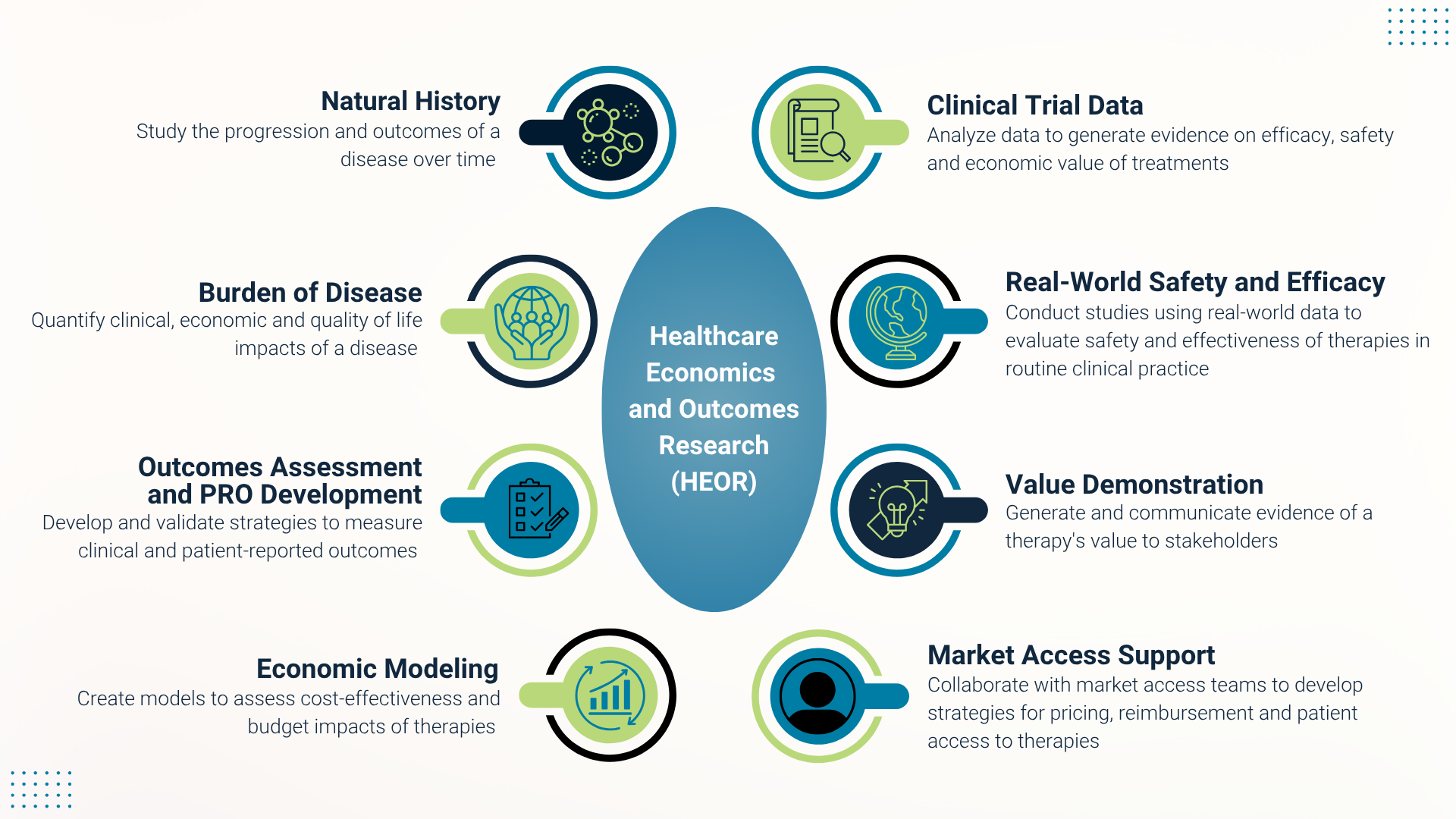
Our Global Health Economics and Outcomes Research (HEOR) and Real-World Evidence (RWE) Team is just one piece of this puzzle, playing an integral role throughout the entire drug development lifecycle from early research to post-approval of a medicine. This work focuses on the collection of real-world insights to help inform trial design, clinical data analysis, real-world performance monitoring, access support and more. The insights gained from these efforts provide key decision-makers – from regulatory authorities to clinicians, to patients and their families – with an accurate, well-rounded view so they can make choices in a timely, effective way.
“I am incredibly proud of Blueprint’s broad commitment to demonstrating the value of our approved medicine in SM to patients, healthcare providers and payers,” said Erin Sullivan, Vice President of Healthcare Economics and Outcomes Research. “Developing patient-reported outcomes tools is one innovative approach we leverage to assess treatment benefit and real-world burden of disease, with the goal of improving the quality of patient care.”
Measuring real experiences: Developing our patient-reported outcomes (PRO) tools for SM
We know how important it is to hear directly from people living with a disease to accurately understand the real-world burden and symptoms they experience. People living with SM face unpredictable and often debilitating symptoms, many of which may be difficult to describe and have important impacts on quality of life. We’ve developed robust, SM-specific patient-reported outcomes (PRO) tools to capture these experiences that are often invisible in lab results but can negatively impact patients’ everyday functioning, work productivity, relationships with family or friends, and even the ability to leave their homes.1,2
Creating these tools requires deep collaboration with the patient community, healthcare providers and others to address the rigorous set of standards. Key steps include:
- Surveying a broad range of patients and clinicians to identify what symptoms are most meaningful to measure
- Ensuring the language used in the questionnaire is easily understood by patients
- Establishing how patient-reported symptom scores correlate with disease severity (e.g., mild, moderate or severe)
- Demonstrating the tool’s ability to detect the effects of a therapeutic intervention
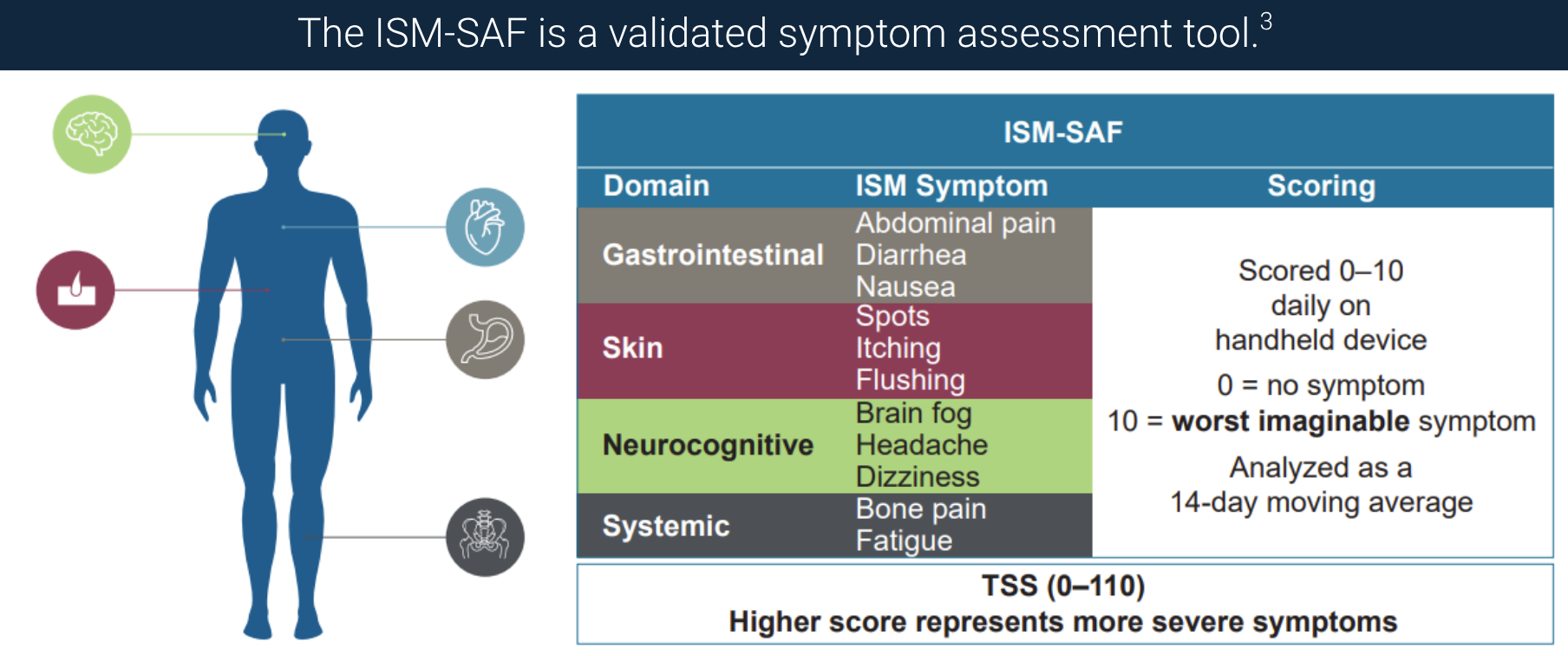
To date, we have developed two SM-specific tools: the Indolent SM Symptom Assessment Form (ISM-SAF) and Advanced SM System Assessment Form (AdvSM-SAF). The ISM-SAF measures symptoms across gastrointestinal, skin and neurocognitive domains, as well as the two additional symptoms of bone pain and fatigue – symptoms identified by patients with ISM as being the most critical to address.
Notably, the mean change in patients’ total symptom score served as an endpoint in our registrational PIONEER clinical trial and was ultimately included in our approved product label, demonstrating the power of patient voices and experiences in shaping regulatory outcomes.
We remain deeply committed to mast cell research and development, including the continued generation of real-world evidence, to advance the understanding of complex diseases and deliver life-changing patient outcomes. To learn more about our HEOR and RWE research, check out our recent presentations and publications.